Abstract
Background: Hydroxyurea (HU) and chronic transfusion therapy (CTT) are the only disease modifying therapies (DMTs) currently available as standard of care in the UK and Italy for patients with sickle cell disease (SCD), with hematopoietic stem cell transplant (HSCT) available to a small fraction of patients. Few registries capture long-term follow up of real-world outcomes among patients with SCD and there is a need for natural history studies demonstrating morbidity and mortality under existing standard of care. The East London Newborn Sickle Cohort Study (ELNSCS) at the Royal London Hospital and the Sickle Cell Disease Cohort (SCDC) at the University of Padova (UNIPD) collect biomarker and clinical data on patients followed comprehensively at two expert referral centers and provide an opportunity to understand SCD real-world outcomes. Here, we report severe acute complications under standard of care during years when HU and CTT were widely available and accepted.
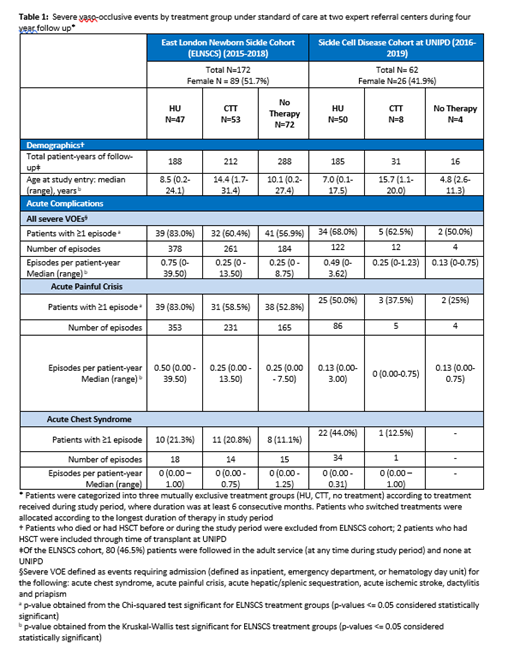
Methods: Inclusion of patients in the ELNSCS required being born in a designated region in London, diagnosed by newborn screening between 1983-2018 and, for this analysis, followed during 2015-2018. Inclusion in the SCDC required being followed, for this analysis, at the UNIPD during 2016-2019. Analyses were restricted to patients with β Sβ S, β Sβ 0, and, for ELNSCS, β Sβ +. Data were entered into clinical databases at each site and subsequently validated against institutional records. Severe vaso-occlusive events (VOEs) were defined as events requiring admission (inpatient, ED, or hematology day unit) for acute chest syndrome (ACS), acute painful crisis, acute hepatic/splenic sequestration, acute ischaemic stroke, dactylitis and priapism. Statistical analysis was aligned between both sites. Patients were categorized into three mutually exclusive treatment groups (HU, CTT, no treatment) according to treatment during study period.
Results: One hundred seventy-two patients in the ELNSCS and 62 patients at UNIPD met study inclusion criteria in the four-year analysis period. HbSS genotype accounted for 161 (93.6%) of ELNSCS cohort and 58 (93.5%) of UNIPD cohort. Median age at study entry was 11.6 (range 0.2 - 31.4) years in the ELNSCS and 7.66 (range 0.12 - 19.96) years at UNIPD. Median age differed across treatment groups; HU group tended to be younger, while CTT group tended to be older.
According to institutional standards of care, 47 (27.3%) patients from the ELNSCS and 50 (80.6%) from UNIPD received HU, 53 (30.8%) from the ELNSCS and 8 (12.9%) from UNIPD received CTT, and 72 (41.9%) from the ELNSCS and 4 (6.5%) from UNIPD received no treatment during the four year study period. Differences in treatment allocation reflect slightly different patient populations and approaches to care at the two centers.
Severe VOEs persist among patients in all three treatment groups at both sites. Of those receiving HU, 83.0% from the ELNSCS and 68.0% at UNIPD had ≥1 severe VOE, including 21.3% from the ELNSCS and 44.0% at UNIPD experiencing ≥1 ACS event. The rate of severe VOEs in the HU group was 0.75 (range 0 - 39.5) per patient year from the ELNSCS and 0.49 (range 0 - 3.00) per patient year from UNIPD. HU dosing and adherence will be explored using data collected on hematologic parameters.
In the ELNSCS, of those receiving CTT, 60.4% experienced ≥1 severe VOE (20.8% had ≥1 ACS event); of those receiving no therapy, 56.9% experienced ≥1 severe VOE (11.1% had ≥1 ACS event). At UNIPD, severe VOEs were observed in 62.5% of the CTT group and 50% of the no treatment group, though sample sizes were very small.
Conclusions: Despite receiving expert care in accordance with local and international guidelines at two large academic centers, a significant sub-group of patients continue to experience severe VOEs. Results show that real-world usage of HU and CTT may not be optimized and, even if optimized, some patients may continue to experience severe acute complications including ACS. Both cohorts confirm that implementation of existing standard of care is insufficient to prevent significant morbidity in patients with SCD. Findings suggest the need to introduce DMT early in life to reduce and prevent acute complications and minimize disease progression. There is a persistent need for maximizing effective DMTs, as well as further developing curative therapies such as HSCT and gene therapy for both pediatric and adult patients.
Colombatti: Novartis: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; BlueBirdBio: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chawla: BlueBirdBio: Current Employment. Puri-Sharma: BlueBirdBio: Current Employment. Walls: BlueBirdBio: Current Employment. Kommera: BlueBirdBio: Current Employment. Telfer: Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Emmaus: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Addmedica: Membership on an entity's Board of Directors or advisory committees; ApoPharma: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BlueBirdBio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Terumo: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal